**Registration for this site visit is closed as it has reached maximum capacity.**
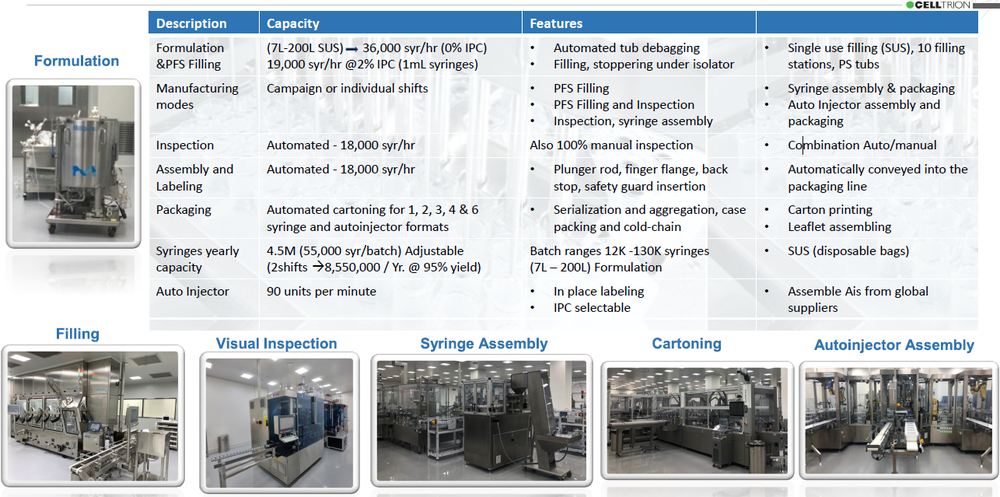
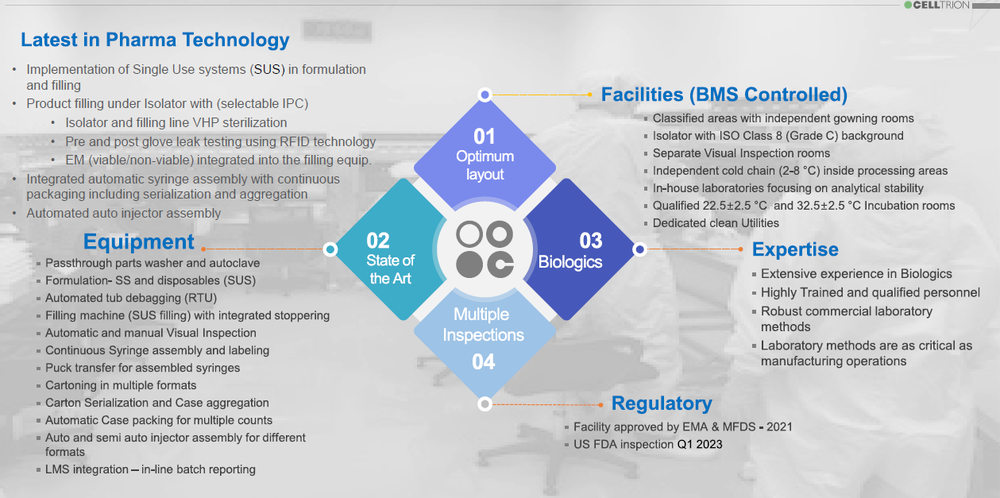
CelltrionPharm Bio Complex is a state-of-the-art facility designed and qualified to fill and package syringes and auto injectors at high speeds. Its CMO services convey the latest innovation in pharmaceutical technology. Implementing single use (SUS) technologies, filling under isolator with integrated EM, automated globe-leak-test using RFID, 100% automatic visual inspection, continuous syringe assembly and labeling, packaging syringes and auto injectors in various formats, and a robotic operated auto injector assembly machine (90upm), considered the fastest in the industry, CelltrionPharm Bio complex represents a globally recognized best-in-class, GMP compliant, Aseptic Filling and packaging of Biopharmaceuticals operations.
The site visit is exclusively free for all registered attendees of the 2022 PDA Aseptic Processing of Biopharmaceuticals Conference.
Please note that pre-registration is required as seats are limited to 40 pax on a first come first serve basis.
State-of-the Art Equipment - Pharma Global Suppliers
Best-In-Class
2022 PDA Aseptic Processing of Biopharmaceuticals Conference
The 2022 PDA Aseptic Processing of Biopharmaceuticals Conference will address topics related to the manufacturing of sterile drug products and patient-friendly applications. This year's conference will build up on last year's theme including topics involving challenges in isolators, glove leak testing, pre filled syringe installations, use of robotics in aseptic filling, and recent innovations in visual inspections. It will also introduce Industry 4.0 in Aseptic filling, advances in glass technologies and Quality in Biotherapy Production.
Our regulatory section will include the participation of MFDS, India and ex-US FDA inspectors providing Regulatory information about changes that will affect the pharmaceutical manufacturing environment and also how to manage regulatory inspections.
Simultaneous Interpretation/Translation (SI) English - Korean will be available

